How do you define nephrotic syndrome?
Nephrotic syndrome Clinical syndrome de fi ned by
(1) large proteinuria of 3 to 4+ by dipstick or Upc >2 g/g creatinine (>0.2 g/mmol) or >4 mg/m 2 /h in a timed urine specimen,
(2) hypoalbuminemia <25 g/l, and
(3) generalized edema
What is remission in NS?
Urinary remission
Urine albumin nil or trace (or proteinuria <4 mg/m 2 /h) for three consecutive days
What is Relapse NS?
Urine albumin 3+ or 4+ (or proteinuria >40 mg/m 2 /h) for three consecutive days, having been in remission previously
WHAT IS Frequent relapses (FRNS) ?
Two or more relapses during 6 months, or more than three relapses during any 12-month period
What is Glucocorticoid (steroid) dependence (SDNS)?
Two consecutive relapses during alternate-day prednisone therapy or within 14 days of its discontinuation
What is the pathology in NS?
Pathology o f Idiopathic Nephrotic Syndrome (INS) The common histopathological variety seen in children with idiopathic nephrotic
syndrome is “minimal change disease” (MCD) or minimal change (or minimal lesion) nephrotic syndrome (MCNS; both terms are used interchangeably). Less commonly seen are focal segmental glomerulosclerosis (FSGS), membranoprolif-erative (or mesangiocapillary) glomerulonephritis (MPGN or MCGN), or (rarely) membranous nephropathy (MN) as causes of nephrotic syndrome.
MCNS is characterized by the lack of histological changes by bright- fi eld and • immuno fl uorescence microscopy.
Electron microscopy reveals generalized effacement of podocyte foot processes.
The term FSGS refers to sclerotic lesions that initially affect some glomeruli • while sparing others (“focal”). Glomeruli are partially affected (“segmental” sclerosis), but the lesions may progress to global sclerosis with progressive nephron loss.
What is the role of podocyte?
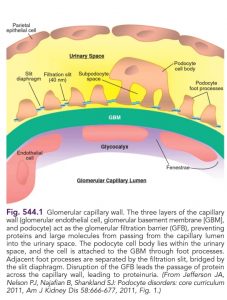
Role of the Podocyte T he underlying abnormality in nephrotic syndrome is an increased permeability of the glomerular capillary wall, which leads to massive proteinuria and hypoalbuminemia.
The podocyte plays a crucial role in the development of proteinuria and the progression of glomerulosclerosis (Fig. 545.2). The podocyte is a highly differentiated epithelial cell located on the outside of the glomerular capillary loop. Foot processes are extensions of the podocyte that terminate on the glomerular basement membrane. The foot processes of a podocyte interdigitate with those from adjacent podocytes and are connected by a slit called the slit diaphragm.
The podocyte functions as structural support of the capillary loop, is a major component of the glomerular filtration barrier to proteins, and is involved in synthesis and repair of the glomerular basement membrane. The slit diaphragm is one of the major impediments to protein permeability across the glomerular capillary wall. Slit diaphragms are not simple passive filters; they consist of numerous proteins that contribute to complex signaling pathways and play an important role in podocyte function.
Important component proteins of the slit diaphragm include nephrin, podocin, CD2AP, and α-actinin 4. Podocyte injury or genetic mutations of genes producing podocyte proteins may cause nephrotic-range proteinuria
What are the clinical presentations?
Clinical Features
T he idiopathic nephrotic syndrome is more common in males than in females (2 : 1) and most commonly appears between the ages of 2 and 6 yr
MCNS is present in 85–90% of patients < 6 yr of age. In contrast, only 20–30% of adolescents who present for the first time with nephrotic syndrome have MCNS.
The more common cause of idiopathic nephrotic syndrome in this older age-group is FSGS. FSGS is the most common cause of end-stage renal disease in adolescents. T he incidence of FSGS has been increasing; African Americans are considered an especially at-risk population.
T he initial episode of idiopathic nephrotic syndrome, as well as subsequent relapses, may follow minor infections and, uncommonly, reactions to insect bites, beestings, or poison ivy.
Children usually present with mild edema, which is initially noted around the eyes and in the lower extremities. Nephrotic syndrome can initially be misdiagnosed as an allergic disorder because of the periorbital swelling that decreases throughout the day.
With time, the edema becomes generalized, with the development of ascites, pleural effusions, and genital edema. Anorexia, irritability, abdominal pain, and diarrhea are common.
Important features of minimal change idiopathic nephrotic syndrome are the absence of hypertension and gross hematuria (the so-called nephritic features).
T he differential diagnosis of the child with marked edema includes protein-losing enteropathy, hepatic failure, heart failure, acute or chronic glomerulonephritis, and protein malnutrition.
A diagnosis other than MCNS should be considered in children < 1 yr of age, with a positive family history of nephrotic syndrome, and/or the presence of extrarenal f indings (e.g., arthritis, rash, anemia), hypertension or pulmonary edema, acute or chronic renal insufficiency, and gross hematuria.
What are the investigations?
Confirming the Diagnosis of Nephrotic Syndrome. The diagnosis of nephrotic syndrome is confirmed by urinalysis with the first morning urine protein:creatinine ratio and serum electrolytes, blood urea nitrogen, creatinine, albumin, and cholesterol levels;
Mx,cxr to rule out tb
an evaluation to rule out secondary forms of nephrotic syndrome (children ≥ 10 yr) by the complement C3 level, antinuclear antibody, double-stranded DNA; hepatitis B and C and HIV in high-risk populations; and kidney biopsy (for children ≥ 12 yr, who are less likely to have MCNS). T he urinalysis reveals 3+ or 4+ proteinuria, and microscopic hematuria is present in 20% of children. A spot urine protein:creatinine ratio should be > 2.0.
The serum creatinine value is usually normal, but it may be abnormally elevated if there is diminished renal perfusion from contraction of the intravascular volume. The serum albumin level is < 2.5 g/dL, and serum cholesterol and triglyceride levels are elevated. Serum complement levels are normal.
A renal biopsy is not routinely performed if the patient fits the standard clinical picture of MCNS.
What is treatment?
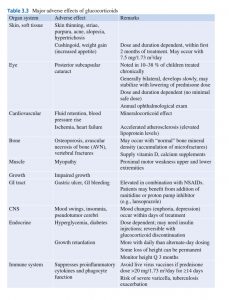
Treatment of the Initial Episode of Nephrotic Syndrome In children with presumed MCNS, prednisone or prednisolone should be administered as a single daily dose of 60 mg/m2/day or 2 mg/kg/day to a maximum of 60 mg daily for 4-6 wk followed by alternate-day prednisone (starting at 40 mg/m2 qod or 1.5 mg/kg qod) for a period ranging from 8 wk to 5 mo, with tapering of the dose. The issue of the duration of steroid treatment has been controversial.
Prolonged steroid treatment with a tapering schedule for 2-5 mo is advocated for decreasing the incidence of relapse based on recent multicenter trials. The Kidney Disease Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group recommends at least 12 wk of steroid treatment. When planning the duration of steroid therapy, the side effects of prolonged corticosteroid administration must be kept in mind. Approximately 80–90% of children respond to steroid therapy.
Definitions regarding the response to steroid therapy are as follows: Response is defined as the attainment of remission within the initial 4 wk of corticosteroid therapy.
Remission consists of a urine protein:creatinine ratio of < 0.2 or < 1+ protein on urine dipstick testing for 3 consecutive days. Most children with minimal change disease respond to daily prednisone therapy fairly quickly, within the first 2-3 wk of treatment.
Relapse is an increase in the first morning urine protein:creatinine ratio > 0.2 or a reading of 2+ and higher for 3 consecutive days on Albustix testing.
Frequently relapsing is two or more relapses within 6 mo after the initial therapy or four relapses in a 12-mo period. Steroid dependent is a relapse during steroid tapering or a relapse within 2 wk of the discontinuation of therapy. Steroid resistance is the inability to induce remission within 4 wk of daily steroid therapy.
Relapse Treatment
• About 70 % of patients with INS will have one or more relapses. Treatment is directed to suppress proteinuria and restore normal serum protein concentrations and to reduce the frequency of future relapses, both with minimal short- and long-term adverse effects
Prednisone remains the only medication to effect remission quickly in patients with glucocorticoid-sensitive, relapsing INS. Prednisone 60 mg/m 2 per day (see above) usually achieves urinary remission within 7–10 days. Once urine is protein-free for three consecutive days, daily prednisone is switched to a single morning dose of 40 mg/m 2 (~1.5 mg/kg) on alternate days for 4 weeks and then stopped
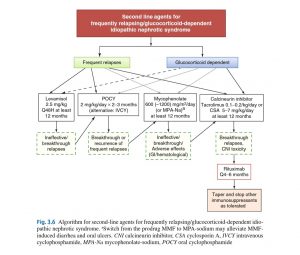
What is frequent relapse?
Frequent Relapses and Glucocorticoid (Steroid) Dependence • • •
Upon achieving remission with daily high-dose prednisone, patient is switched to alternate-day prednisone as above. However, there is no clear consensus about the best long-term approach.
Option 1: identi fi cation of a “threshold” dose of prednisone. Alternate-day prednisone is tapered to 0.5 mg/kg every 48 h. If stable, taper is continued until a dose is reached that still prevents relapses. When a relapse occurs, aim at a maintenance dose just above the last dose where the patient relapsed and continue this dose for 6 months.
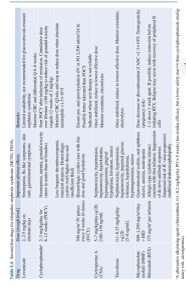
Option 2: introduction of second-line agent, usually in conjunction with prednisone tapering, intended to minimize long-term glucocorticoid adverse effects




 Superintendent, ICH.
Superintendent, ICH.